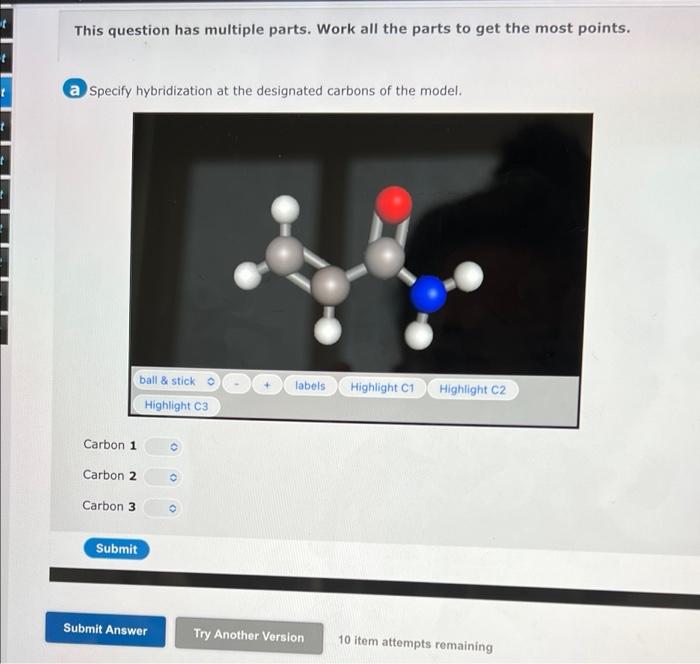
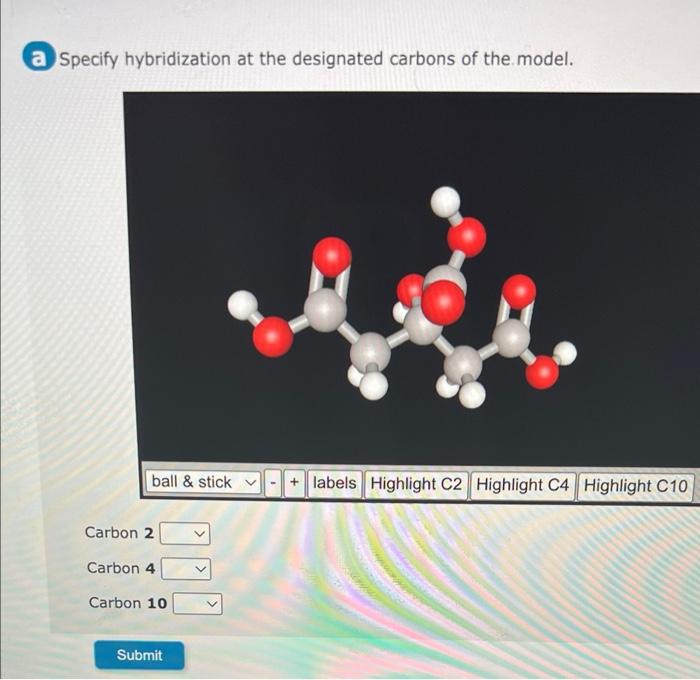
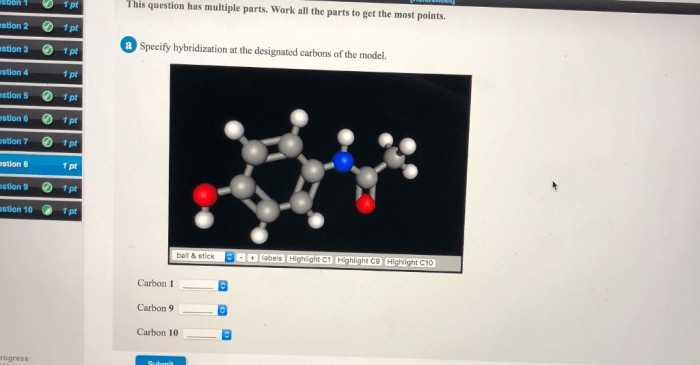
Specify hybridization at the designated carbons of the model is a fundamental concept in chemistry that provides a detailed understanding of molecular structure and properties. By determining the hybridization of specific carbon atoms, chemists can predict and explain a wide range of chemical phenomena.
This concept plays a crucial role in various fields of chemistry, including organic chemistry, inorganic chemistry, and materials science. It enables researchers to design new materials with tailored properties, understand chemical reactions at the molecular level, and develop advanced technologies.
Specify Hybridization at Designated Carbons
Hybridization is the process of combining atomic orbitals to form new hybrid orbitals with different shapes and properties. In a molecule, the hybridization of the carbon atoms determines the geometry of the molecule and its chemical properties.
Designated carbons are specific carbon atoms in a molecule that are assigned a particular hybridization state. The hybridization of a designated carbon atom can be determined by examining the number and type of bonds it forms.
Types of Hybridization
- sp Hybridization:Occurs when a carbon atom forms two single bonds. The hybrid orbitals are linear and 180° apart.
- sp2Hybridization: Occurs when a carbon atom forms three bonds, one of which is a double bond. The hybrid orbitals are trigonal planar and 120° apart.
- sp3Hybridization: Occurs when a carbon atom forms four bonds. The hybrid orbitals are tetrahedral and 109.5° apart.
Methods for Determining Hybridization
Spectroscopic Techniques
Infrared (IR) and nuclear magnetic resonance (NMR) spectroscopy can be used to determine the hybridization of carbon atoms. IR spectroscopy measures the vibrational frequencies of bonds, which can provide information about the hybridization of the atoms involved. NMR spectroscopy measures the chemical shifts of nuclei, which can also provide information about hybridization.
Computational Methods
Computational methods, such as density functional theory (DFT), can be used to predict the hybridization of carbon atoms. These methods use quantum mechanics to calculate the electronic structure of molecules, which can provide information about the hybridization of the atoms.
Interpreting Experimental Data
To determine the hybridization of a designated carbon atom, experimental data from spectroscopic or computational methods must be interpreted. This involves comparing the experimental data to known values for different hybridization states.
Impact of Hybridization on Molecular Properties
Bond Lengths and Bond Angles
Hybridization influences the bond lengths and bond angles in a molecule. For example, sp 3hybridized carbon atoms form tetrahedral bonds, which are longer and less strong than sp 2hybridized carbon atoms, which form trigonal planar bonds.
Molecular Polarity
Hybridization can also affect the polarity of a molecule. Polar molecules have a separation of charge, with one end of the molecule being more positive and the other end being more negative. Sp 3hybridized carbon atoms tend to form nonpolar bonds, while sp 2and sp hybridized carbon atoms can form polar bonds.
Molecular Reactivity
Hybridization can also influence the reactivity of a molecule. For example, sp 2hybridized carbon atoms are more reactive than sp 3hybridized carbon atoms. This is because sp 2hybridized carbon atoms have a higher energy and are more likely to react with other molecules.
Applications of Hybridization in Chemistry: Specify Hybridization At The Designated Carbons Of The Model
Designing New Materials, Specify hybridization at the designated carbons of the model
Hybridization is used in the design of new materials with specific properties. For example, carbon nanotubes are made from sp 2hybridized carbon atoms and have unique electrical and mechanical properties.
Understanding Chemical Reactions
Hybridization is essential for understanding chemical reactions. The hybridization of the reactants and products determines the reaction pathway and the rate of the reaction.
Various Fields of Chemistry
Hybridization is applied in various fields of chemistry, including organic chemistry, inorganic chemistry, and biochemistry. In organic chemistry, hybridization is used to explain the structure and reactivity of organic molecules. In inorganic chemistry, hybridization is used to explain the structure and bonding of inorganic compounds.
In biochemistry, hybridization is used to explain the structure and function of biological molecules.
Popular Questions
What is hybridization?
Hybridization is the process of combining atomic orbitals to form new hybrid orbitals with different shapes and energies.
What are designated carbons?
Designated carbons are specific carbon atoms in a molecule whose hybridization is of interest.
How can we determine the hybridization of carbons?
Hybridization can be determined using spectroscopic techniques (e.g., IR, NMR) or computational methods.