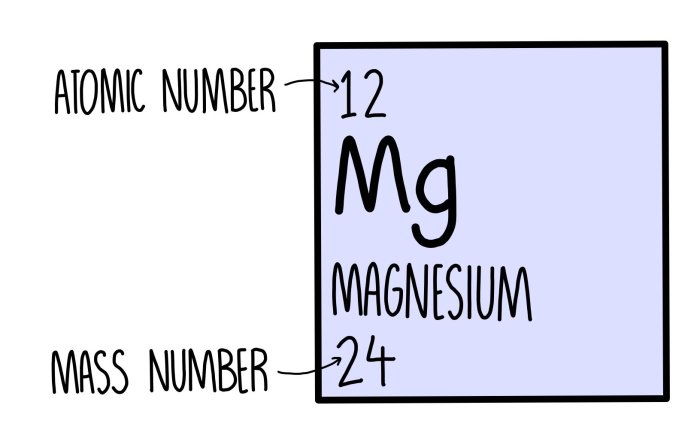The chemistry atomic number and mass number worksheet delves into the fundamental concepts of atomic structure, providing a comprehensive understanding of the properties and characteristics of elements. This guide explores the atomic number, mass number, and their intricate relationship, empowering learners to identify and analyze elements with precision.
Delving deeper into the topic, the worksheet delves into the relationship between atomic number and mass number, uncovering how these fundamental properties determine an element’s identity. Through engaging exercises and thought-provoking questions, learners will gain a profound understanding of the building blocks of matter.
Atomic Number and Mass Number: Chemistry Atomic Number And Mass Number Worksheet
Atomic number and mass number are two fundamental properties that define the identity of an element. Understanding these concepts is crucial for comprehending the structure and behavior of atoms.
Atomic Number
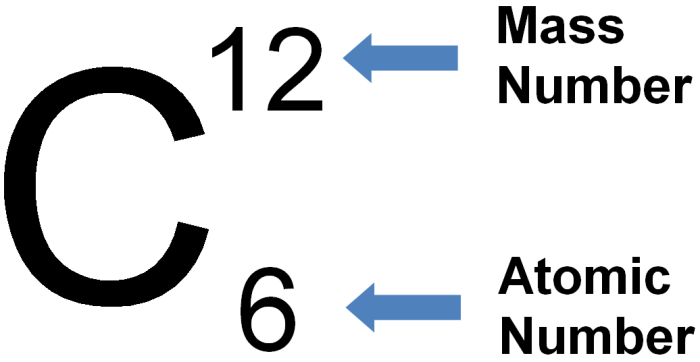
The atomic number of an element is the number of protons in its nucleus. Protons carry a positive electric charge, and the number of protons determines the element’s chemical properties and position on the periodic table.
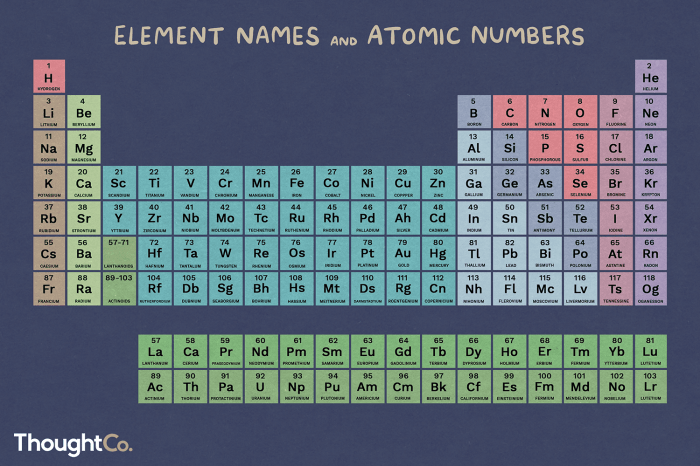
To find the atomic number of an element, refer to the periodic table. Each element is assigned a unique atomic number, which is typically displayed above the element’s symbol.
| Element | Symbol | Atomic Number |
|---|---|---|
| Hydrogen | H | 1 |
| Carbon | C | 6 |
| Oxygen | O | 8 |
Mass Number
The mass number of an element is the total number of protons and neutrons in its nucleus. Neutrons are subatomic particles with no electric charge and contribute to the element’s mass.
To find the mass number of an element, refer to the periodic table. The mass number is typically displayed below the element’s symbol.
| Element | Symbol | Mass Number |
|---|---|---|
| Hydrogen | H | 1 |
| Carbon | C | 12 |
| Oxygen | O | 16 |
Relationship between Atomic Number and Mass Number, Chemistry atomic number and mass number worksheet
The atomic number and mass number of an element are closely related. The atomic number determines the element’s chemical identity, while the mass number provides information about the number of protons and neutrons in the nucleus.
Together, the atomic number and mass number uniquely identify an element. For example, all atoms with an atomic number of 6 are carbon atoms, regardless of their mass number.
The number of neutrons in an atom can vary, resulting in isotopes of the same element. Isotopes have the same atomic number but different mass numbers.
Commonly Asked Questions
What is the atomic number of an element?
The atomic number of an element is the number of protons in its nucleus.
How do I find the mass number of an element?
The mass number of an element is the sum of the number of protons and neutrons in its nucleus.
What is the relationship between atomic number and mass number?
The atomic number and mass number of an element determine its identity and position on the periodic table.